Chimeric antigen receptor (CAR) T cells, genetically altered immune cells extracted from patients and subsequently reintroduced, have obtained notoriety for treating blood malignancies. However, is it possible to utilize these cells to combat conditions like aging?
In a study published in the journal Nature, Lowe and colleagues from Sloan Kettering Institute in New York demonstrated the effects of CAR T cells against various diseased tissues. Senescence, a state of cell arrest and an inducer of elevated inflammation, negatively affected the diseased tissues used in the study. This potential therapeutic may one day be utilized to treat a variety of conditions linked to senescence, such as diabetes, atherosclerosis, and fibrotic liver disease.
CAR T Cells Need Targets to Locate and Destroy Diseased Cells
When utilized to treat cancer, doctors extract immune cells called T cells from a patient and genetically modify them to target cancer cells prior to redelivering them to the body. Upon reintroduction of the cells into the patient, the CAR T cells accurately and precisely pinpoint and eliminate cancer cells.
However, a proper target is needed for the cells to utilize CAR T cell therapy successfully. Correspondingly, CD19, a blood cancer cell-surface protein seen in malignant cells and few healthy cells, became the first FDA-approved target for CAR T cells. Based on this previous research, Lowe and colleagues aimed to locate a molecular target on senescent cells.
“Senescence is a double-edged sword,” said Scott Lowe, Ph.D., a senior author on the paper in a press release. “Cells in this state play an important role in wound healing and cancer deterrence. But if they linger for too long, they can cause chronic inflammation, which itself is a cause of many diseases. Finding a way to safely eliminate these cells would be a major therapeutic breakthrough in the treatment of these diseases.”
In terms of the significance of senescent cells in disease, the authors wrote, “the aberrant accumulation of senescent cells generates an inflammatory milieu that leads to chronic tissue damage and contributes to diseases such as liver and lung fibrosis, atherosclerosis, diabetes, and osteoarthritis.” They went on to say that removing senescent cells from injured mouse tissue “ameliorates the symptoms of these pathologies and even promotes longevity.”
Senescent Cells Exclusively Present the Receptor uPAR
The investigators compared compounds on the surface of senescent cells to various cells types to find a molecular characteristic that differentiates senescent cells from other types of cells. As a result, they discovered a compound called urokinase plasminogen activator receptor (uPAR) concentrated on these cells. Other cell types typically don’t contain uPAR molecules. To locate this cell surface marker, investigators promoted senescence in some cells and proceeded with the examination of those cells. They quantified the amount of present ribonucleic acid (RNA), a polymeric compound indicative of protein expression, to differentiate the protein levels present in senescent cells and other cell types. As a result, they accurately and precisely located uPAR on the cell surface of cells exhibiting senescence.
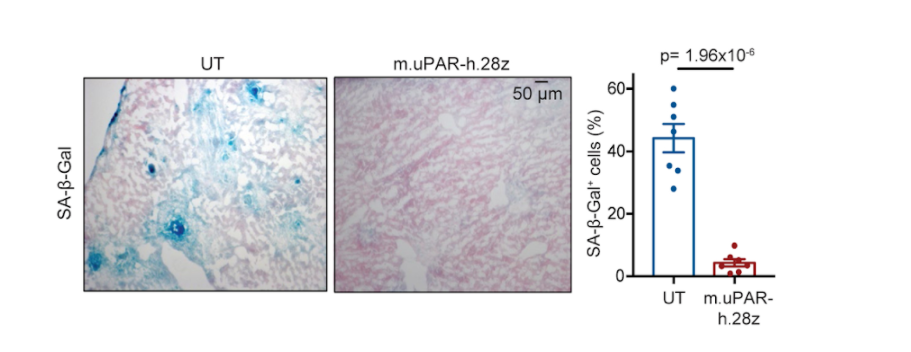
The investigators then generated CAR T cells that detect uPAR, allowing the modified immune cells to target senescent cells that express uPAR. To determine whether the genetically engineered CAR T cells were capable of killing senescent cells, the researchers looked at whether these immune cells would target and dispose of senescent liver cells that were genetically induced (oncogene-induced senescence). Within ten days of treatment, investigators noted that senescent cells were effectively cleared. Furthermore, results demonstrated that the engineered CAR T cells could eradicate senescent cells in laboratory dishes.
To determine whether the proposed treatment was successful in live animals, investigators treated live mice with various diseases that induce senescence utilizing these CAR T cells. They modeled lung cancer using a disease mouse model, and results showed prolonged survival and diminished levels of lung tumor cells upon treatment with CAR T cells.
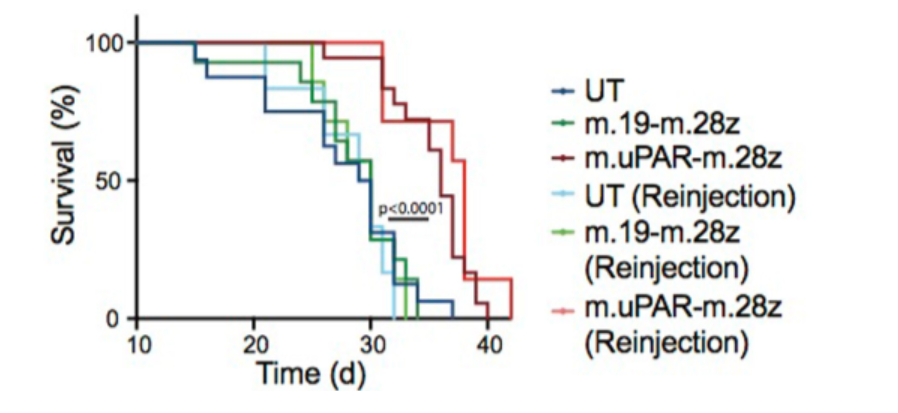
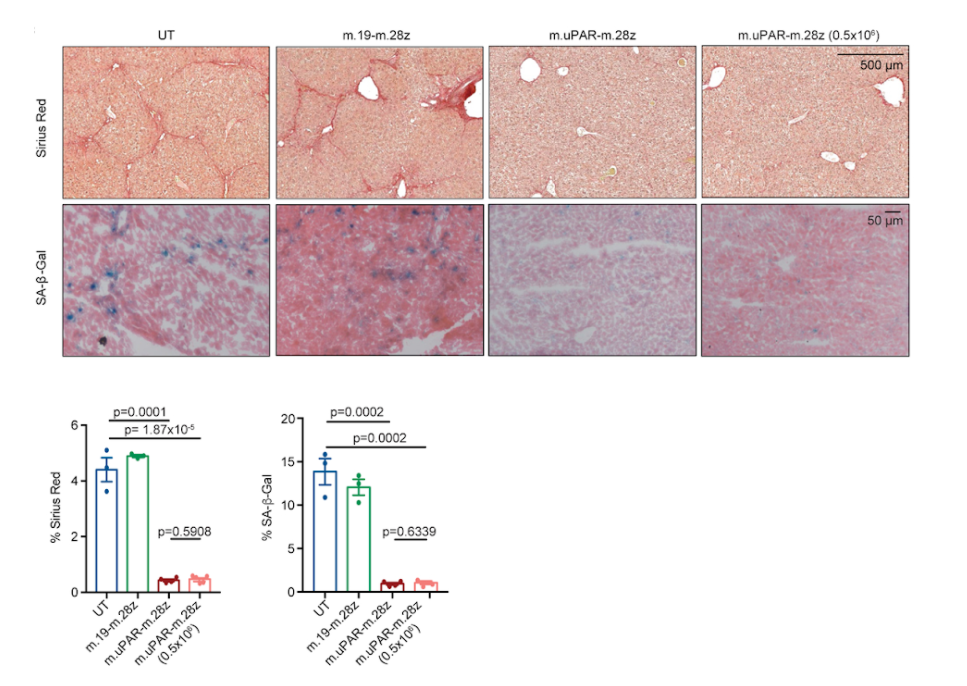
Investigators also looked at how well the CAR T treatment worked in some models of liver fibrosis, which induces cell senescence. Liver fibrosis was induced in mice using carbon tetrachloride, and results showed that upon treatment with CAR T cells, the mice exhibited diminished levels of senescent cells. In addition, mice with non-alcoholic steatohepatitis, a form of diet-induced liver fibrosis, demonstrated significantly lower levels of senescent cells upon undergoing CAR T cell treatment that targeted uPAR.
The experiments showed that CAR T cell treatment successfully eliminated senescent cells in both laboratory dishes and live mice. “This study demonstrates that T cell engineering and CAR therapy can be effective beyond cancer immunotherapy,” said Michael Sadelain, MD, Ph.D., an author of the study in a press release.
Applying CAR T Cells to Other Age-Related Diseases
The next course of action will be to determine whether uPAR-targeting T cells can treat other diseases linked to senescence, such as diabetes, osteoarthritis, and atherosclerosis. The investigators of this study aspire to eventually treat people with CAR T therapy.
“We think this approach has the potential to tackle a number of senescence-related diseases for which new treatments are badly needed,” added senior author Scott Lowe, Ph.D.