Nicotinamide adenine dinucleotide (NAD+) is an essential energy-producing molecule and plays a crucial role in maintaining DNA integrity. Levels of NAD+ in cells decrease upon aging, and scientists have connected these age-induced decreases in NAD+ to the development of Alzheimer’s disease, metabolic disorders, and cardiovascular dysfunction. CD38 is an enzyme that has been demonstrated to serve an important role in decreased levels of NAD+ upon aging; however, more studies are needed to determine its overall function. Researchers are seeking to elucidate which cells and mechanisms give rise to active CD38 upon aging, in addition to shedding light on the enzyme’s function during NAD+ decline.
The journal Nature Metabolism contains two recent articles that address the connection between CD38 and NAD+. Chini and colleagues from the Mayo Clinic in Minnesota and Covarrubias and colleagues from the Buck Institute for Research on Aging in California each published an article demonstrating how aging-induced CD38 activity in M1 macrophages (immune cells) was linked to inflammation. Degradation of NAD+ in cells and controlled bioavailability of extracellular nicotinamide mononucleotide (NMN), a precursor to NAD+, resulted from CD38 activity and ultimately led to diminishing levels of NAD+ in tissue.
Upon senescence, a state of cell division arrest, a process called senescence-associated secretory phenotype (SASP) induces the secretion of inflammatory factors from senescent cells. These factors increase in presence upon aging and lead to CD38 activity on immune cells. The increased levels of CD38 induce the SASP cellular loop, which is responsible for progressively reducing the levels of NAD+. However, we still need to determine which specific immune cells utilize CD38 and for what purpose.
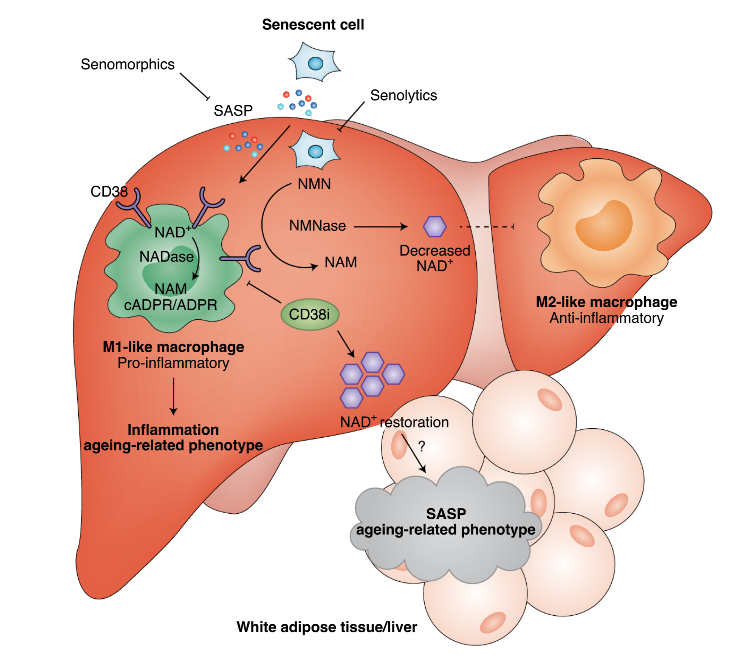
According to the study conducted by Chini and colleagues, M1 macrophages, which have been shown to give rise to inflammation, were shown to exhibit the most CD38 activity among immune cell types in aged tissues. Their results were corroborated by Covarrubias and colleagues, who showed that CD38 consumes NAD+ in M1 macrophages. However, both studies failed to discuss the possible consequences that can arise from CD38 induced consumption of NAD+ by M1 macrophages. Therefore, figuring out why CD38 activity is elevated in M1 macrophages is essential in establishing a better understanding of whether inhibition of CD38 leads to unintended harmful consequences.