T lymphocytes, a type of white blood cell, contribute to the body’s immune response against cancer. T lymphocyte antitumor responses are metabolically challenged when nutrients are depleted from the cellular microenvironment that tumors form, resulting in T cell malfunction. Researchers have yet to elucidate the mechanisms controlling the effects tumors have on T lymphocyte antitumor responses.
In a study published in the journal Nature Immunology, scientists from the University of Lausanne in Switzerland discovered that the tumor’s microenvironment caused decreased fitness of the cell’s energy generator, the mitochondria, in tumor-infiltrating T cells. In addition, the obtained results from this study indicated that mice who underwent nicotinamide riboside (NR) supplementation exhibited elevated T lymphocyte mitochondrial function and improved antitumor response.
Studies have indicated that NR facilitates increased healthspan and lifespan of mice and halts age-related conditions by elevating levels of an essential coenzyme called nicotinamide adenine dinucleotide (NAD+), which is responsible for genomic stability and generating cellular energy. Despite this, scientists have expressed varying and even contradictory opinions on the effects of elevating NAD+ levels on cancer.
According to the published study in Nature Immunology, the accumulation of defective mitochondria, the cell’s powerhouse, leads to abnormalities in tumor-infiltrating T lymphocyte function. In the tumor microenvironment, the cellular area proximal to the tumor, T lymphocytes acquire mitochondria with impaired inner membranes, thin layers that sectionalize the mitochondria, thus implying mitochondrial dysfunction.
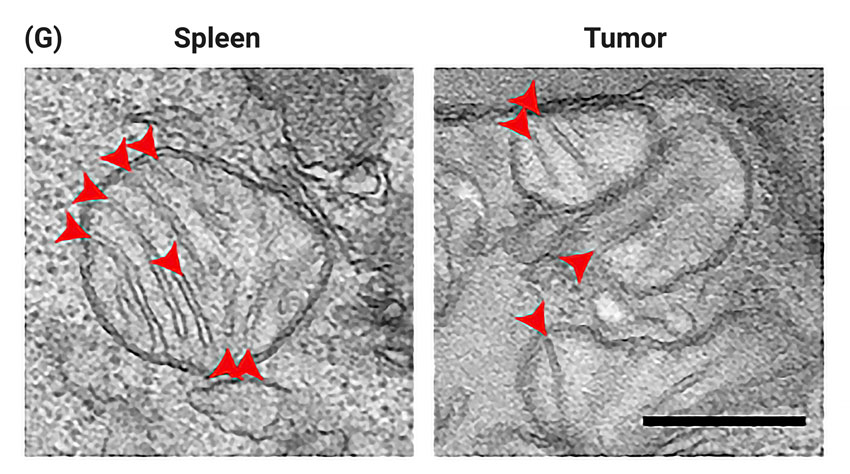
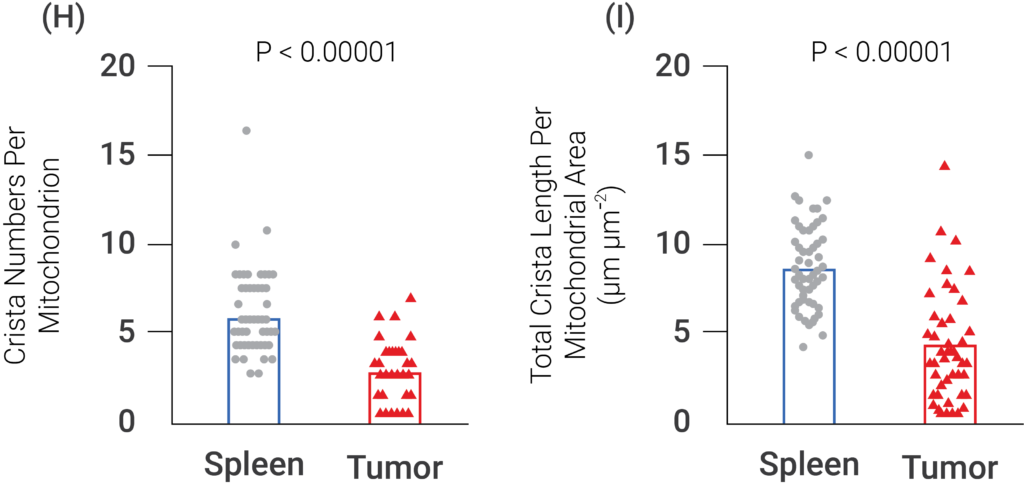
(Yu et al., 2020 | Nature Immunology) The folds of the inner mitochondrial membrane (i.e., cristae) are increased in T lymphocytes present in normal spleens compared to tumors, indicating that the tumor microenvironment caused T cell mitochondrial dysfunction. (G) Cristae numbers and length are increased in the T cells of normal spleens compared to tumors, illustrating that T cell mitochondria function is diminished by the tumor microenvironment. (H) The numbers of the inner membranes, the cristae, are significantly lower in T cells of tumors compared to T cells present in normal spleens. (I) The lengths of the cristae are significantly lower in T cells of tumors compared to T cells in normal spleens.
The team of investigators also discovered that T lymphocytes are driven into a state of terminal exhaustion and become permanently dysfunctional when tumor-infiltrating T lymphocytes acquire too many malfunctional mitochondria. Furthermore, upon transplantation of tumor T cells to the spleen of mice, the investigators noticed that transplanted T cells exhibited a considerable decrease in re-expansion, suggesting that the effector function of the cells was progressively diminishing (i.e., terminal exhaustion). According to these findings, T lymphocytes exhibit diminished immunological function once they’re in a permanent cellular state of terminal exhaustion, which occurs upon exposure to the area proximal to the tumor.
In addition, the investigators of this study demonstrated that supplementing mice with NR induced a boost in mitochondrial function and offered a bit of antitumor immunity. Due to prior research showing that elevating NAD+ levels enhance mitochondrial health, the group of investigators believed that administering mice with NR would activate antitumor immunity and stop the tumor-infiltrating T lymphocytes from becoming cellularly exhausted. Indeed, they discovered that supplementing mice with NR elevated mitochondrial health and increased the impact of PD-1, a checkpoint inhibitor that boosts the T cell response to malignancies, on tumor growth.
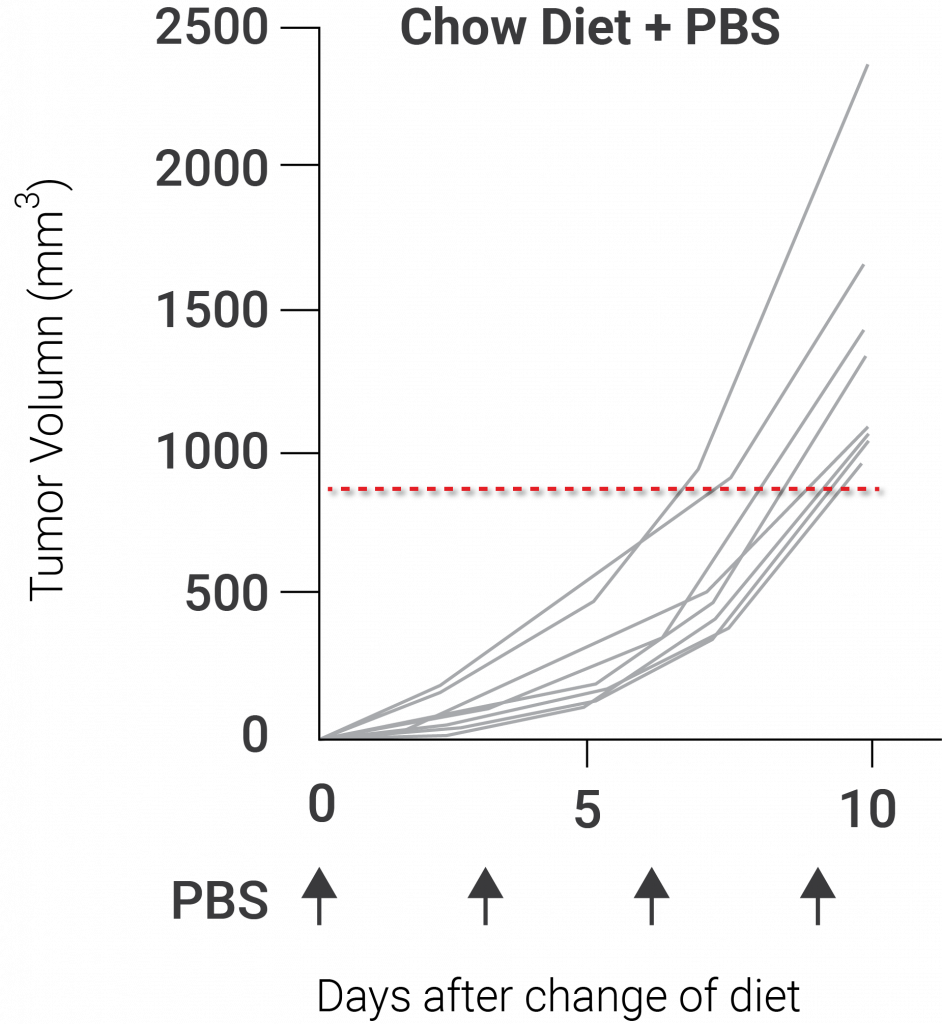
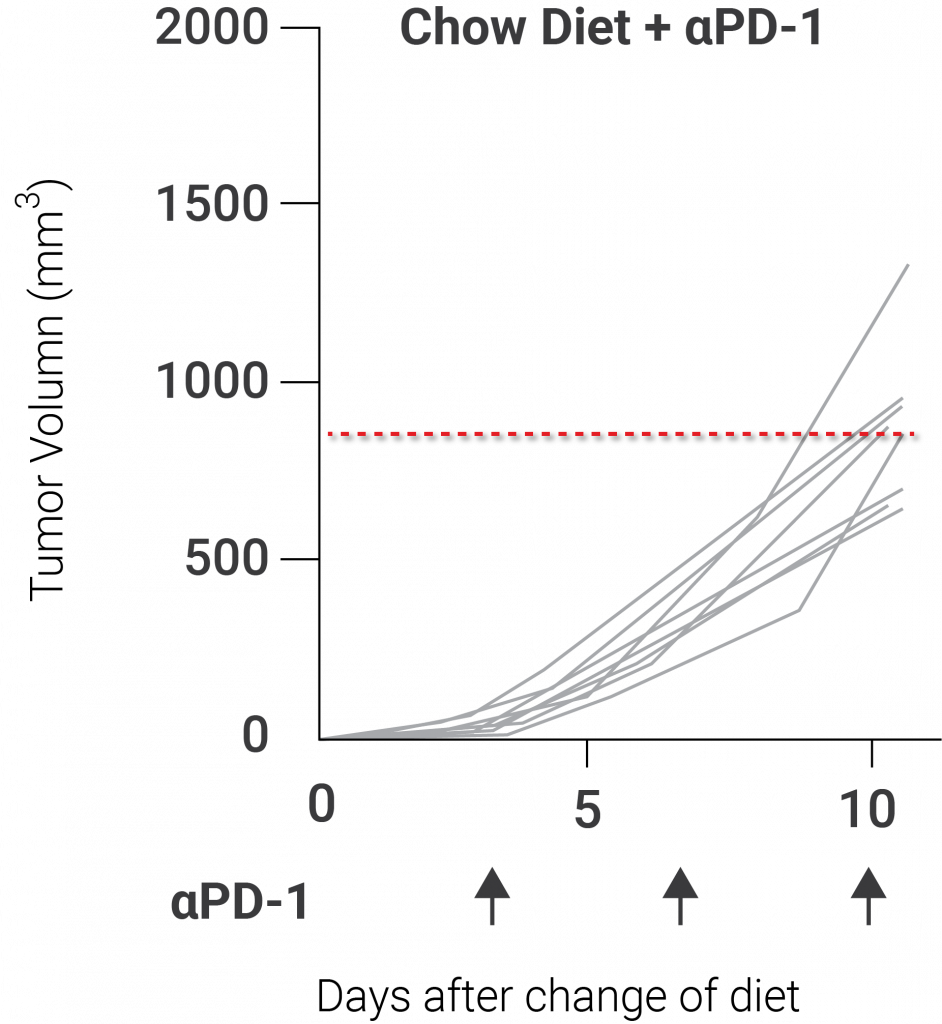
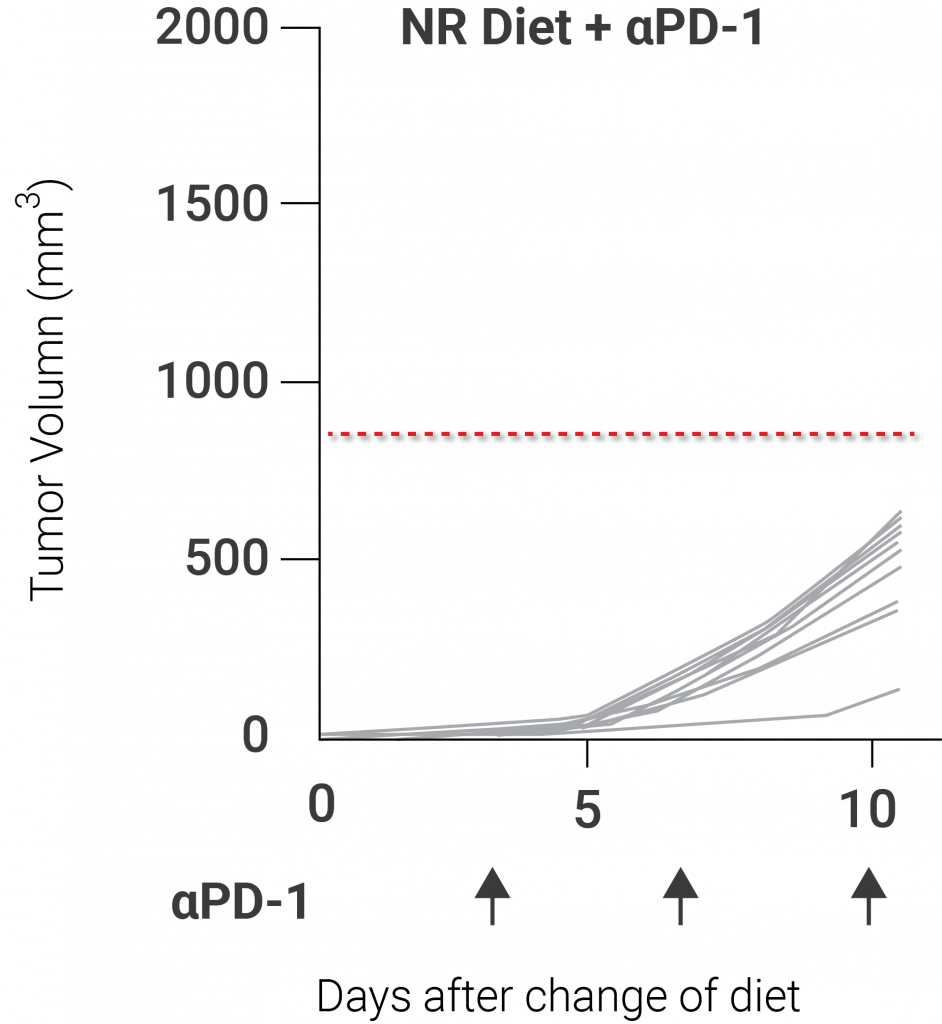
(Yu et al., 2020 | Nature Immunology) Nicotinamide riboside (NR) sustains mitochondrial fitness to enhance antitumor responses in T cells. NR potentially enhances antitumor immunity with treatment with PD-1, a checkpoint inhibitor protein that improves the T cell response to tumors.
Treatment with nicotinamide riboside presents a promising strategy to prevent mitochondrial dysfunction,” stated the investigators in their study. The study’s findings indicate that tumor microenvironments are linked to tumor-infiltrating T lymphocytes acquiring too many malfunctional mitochondria, which induces persistent cellular exhaustion in T cells. Furthermore, these researchers suggest that these studies may contribute vital knowledge that can be used to develop therapeutics that ameliorate T cell antitumor responses.