Coronavirus genomes, which contain the instructions for constructing and assembling the virus, lack the ability to encode enzymes required for energy production and the generation of biological building blocks. Thus, the assembly of these viruses is dependent on the utilization of infected host cell processes. The viral genome-encoded processes for energy production and the generation of chemical molecules rely on the host’s nicotinamide adenine dinucleotide (NAD+) system, which is essential for the optimal function of numerous enzymes in infected cells, including those involved in antiviral defense. However, we have yet to properly investigate how the host NAD+ system and the enzymes that rely on it are taken over for viral replication or utilized as antiviral defenses.
In an article published in the Journal of Biological Chemistry, Heer and colleagues from the University of Iowa demonstrate that SARS-CoV-2 infection notably upregulates a subset of antiviral compounds that rely on NAD+ called non-canonical Poly-ADP-ribose polymerases (PARPs) and promotes gene expression that leads to the encoding of nicotinamide riboside (NR) and a form of vitamin B3 called nicotinamide (NAM), both of which are precursor enzymes that promote NAD+ synthesis. In addition, they showed that other NAD+ biosynthetic pathways are downregulated upon infection from SARS-CoV-2. Furthermore, they demonstrate that NR and NAM supplementation significantly reduced the replication of a PARP-sensitive mouse coronavirus. The presented data suggest that innate immunity to coronaviruses could be enhanced via nutritional and pharmacological interventions that elevate the levels of NAD+.
To obtain a better understanding of the link between NAD+ and coronaviruses, Heer and colleagues examined human cell lines infected with SARS-CoV-2, a lung biopsy from humans living with or dead from COVID-19, and bronchoalveolar lavage fluid from healthy patients in addition to those infected with COVID-19. The investigators demonstrate that SARS-CoV-2 infections activate the genes that encode enzymes for the salvage pathway of NAD+ synthesis from nicotinamide (NAM) and nicotinamide riboside (NR) while diminishing the generation of enzymes for other NAD+ biosynthetic pathways, including those that use nicotinic acid (NA). In addition, they found that SARS-CoV-2 infections led to non-canonical PARP compounds consistently exhibiting elevated protein production.
The team of investigators from the University of Iowa demonstrates that the activity of these non-canonical PARP compounds is restricted by cellular NAD+ and can be augmented by pharmacological stimulation of NAD+ synthesis. Furthermore, they show that infection with a mouse variant of coronavirus causes an extreme attack on NAD+ within the host cell. These findings suggest that NAD+ enhancing agents should be tested in the setting of CoV infections. On top of animal trials, the safety of different forms of vitamin B3 should enable fast clinical assessments of NAD+ boosters to be investigated.
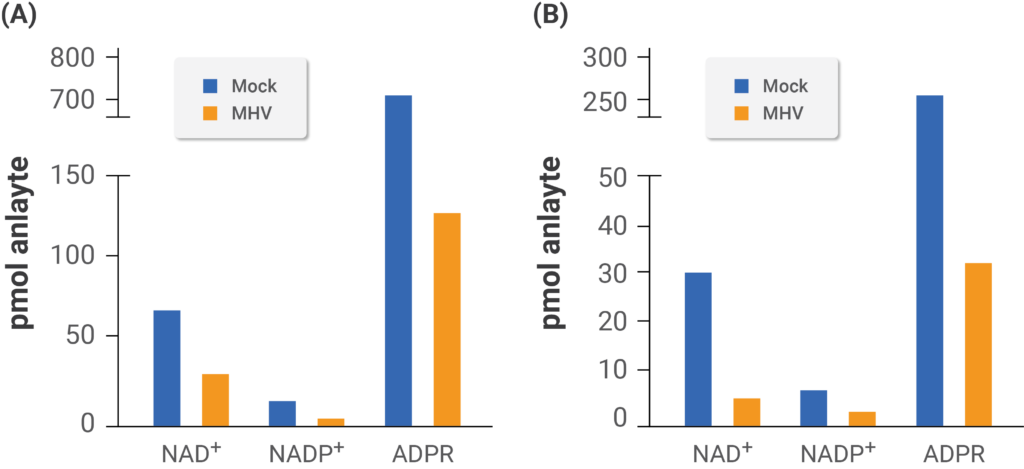
(Heer et al., 2020 | Journal of Biological Chemistry) Mouse coronavirus infection disturbs the NAD+ system. The researchers tested levels of NAD+ and related compounds in mouse cells derived from brain tumors (A) and bone marrow (B) infected with mouse coronavirus (MHV). The white bars depict non-infected mouse cells, and the black bars depict mouse cells infected with mouse coronavirus.
The team of researchers then constructed a system to test whether boosted NAD+ status combats mouse coronavirus infection utilizing a mutant strain of the virus with inadequate replication capabilities and is not as deleterious to host cells (i.e., virulent) due to its incapability of blocking the antiviral PARP response.The scientists discovered that NAM and a clinically tested formulation of NR substantially suppressed replication of the less-hostile mutant mouse coronavirus but not the non-mutated strain, confirming their findings that SARS-CoV-2 infection upregulates the pathways for NAD+ production from NAM and NR. In line with their findings that SARS-CoV-2 infection upregulates the NAD+ synthetic pathways from NR and NAM, the investigators discovered that NAM and a clinically tested formulation of NR substantially suppressed replication of the less-detrimental mutant mouse coronavirus; however, this did not occur for the non-mutated strain. Thus, Heer and colleagues hypothesize that NAD+ enhancing producers involving elevated NA-dependent synthesis were not likely to be highly efficient because SARS-CoV-2 infection diminished the concentration of host enzymes involved in that pathway for NAD+ synthesis.
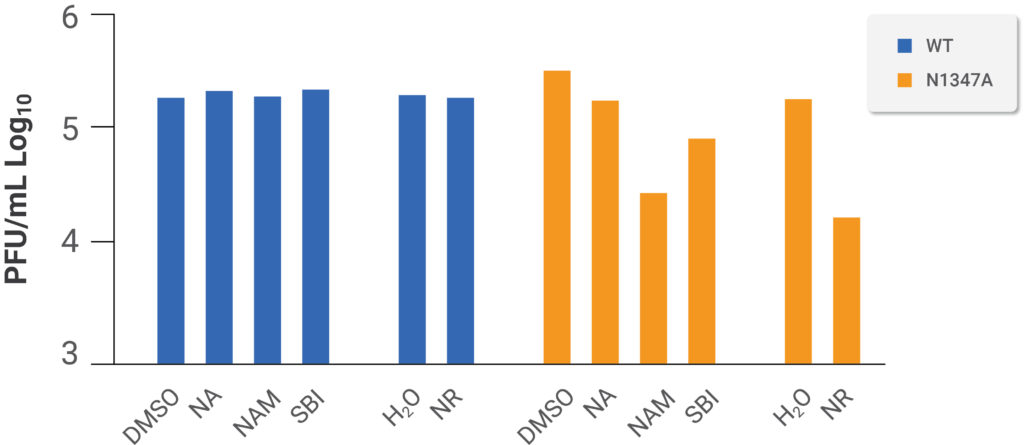
(Heer et al., 2020 | Journal of Biological Chemistry) Boosting NAD+ levels with NAM and NR depresses the replication of a mutant mouse coronavirus with limited replication ability and virulence but does not affect unmutated mouse coronavirus. Mouse cells were infected with either unmutated (WT) or mutated (N1347A) mouse coronavirus with limited ability to replicate and harm the host cell and either mock-treated (DMSO or H2O) and treated with NA, NAM, NR, or an activator of an enzyme that participates in the generating NAD+ (SBI). These results show that NAM, NR, and SBI have anti-viral effects in a mutant form of mouse coronavirus that isn’t as harmful to host cells as naturally occurring mouse coronaviruses.
Furthermore, these findings indicate that the antiviral activities of non-canonical PARP enzyme activities are restricted to NAD+ bioavailability and that innate immunity to coronaviruses may be ameliorated by elevating NAD+ levels through pharmacological and nutritional interventions. Moreover, the findings support further investigation into how pharmacological and nutritional modification of NAD+ levels may potentially limit viral infection by increasing antiviral PARP activity.
“While caution should be exercised with respect to any preventative measure, NAD+ boosting approaches have the potential to support the innate immune system and address the age-, smoking-, and comorbid conditions associated with worse SARS-CoV-2 outcomes,” said the investigators in the article.
Since there is no available standard of care for caretakers or housemates of infected individuals, the investigators propose that NAD-boosting might be explored as a protective strategy against infection for those living in close proximity to isolated or hospitalized COVID-19 patients. The potential societal value of a safe and easily accessible compound to aid prevention and public health cannot be overstated, especially as novel COVID-19 outbreaks surface.