The transferring of electrons via metabolic pathways is essential to produce the energy required for human cells to function. To preserve this vital energy-producing process, electrons bind to nicotinamide adenine dinucleotide (NAD+), a crucial compound for cellular metabolism, and convert it to NADH. However, as humans age, the concentration of electrons and the ratio of NAD+ to NADH become disproportionate, which compromises the cell’s homeostasis—the preservation and regulation of the stability and consistency required for appropriate cell function. Thus, these cell modifications may play a key role in metabolic dysfunction, which aids the progression of age-related disorders.
In an article published in Aging, Baur and colleagues from UPenn indicated that treatment with rapamycin averts diminished NAD+ to NADH ratios in the muscle of mice. The presented findings show that rapamycin prefers a greater NAD+ to NADH ratio and enhances energy production. This research adds to our understanding of how rapamycin may alter the aging process and suggests that improper NAD+ to NADH ratios may be a signal that can be targeted utilizing rapamycin to enhance the health and longevity of aging individuals.
How Does Rapamycin Extend Experimental Animal Lifespan?
Studies have demonstrated that rapamycin treatment prolongs lifespan in organisms, such as yeast and mice, and could stimulate healthy aging in individuals. Therefore, Baur and colleagues sought to examine how the NAD+ to NADH ratio is affected by rapamycin to develop a deeper understanding of how rapamycin improves lifespan. According to previous studies, rapamycin inhibits the synthesis of lactate, a compound that converts food into energy and aids the conversion of NAD+ to NADH. By reducing the lactate synthesis, rapamycin may prevent the conversion of NAD+ to NADH and inhibit potential lactate-induced NAD+/NADH electron imbalance. Perhaps rapamycin’s ability to diminish lactate synthesis has an impact on the electron balance NAD+ to NADH ratio, resulting in increased longevity.
Rapamycin Improved Mouse Muscle Cell NAD+/NADH Ratio
To determine whether rapamycin administration elevated the NAD+ to NADH ratio (NAD+/NADH), the investigators utilized myoblasts, which are mouse embryonic precursors of muscle cells that grow into muscle cells. Long-term cell development of myoblasts in laboratory dishes (cell culture) with 100 nM rapamycin notably raised NAD+/NADH concentrations while decreasing NADH concentrations. Because their findings showed that rapamycin administration had no effect on NAD+ concentrations, Baur and colleagues concluded that the reduced NADH concentrations induced the alterations in NAD+ to NADH concentrations.
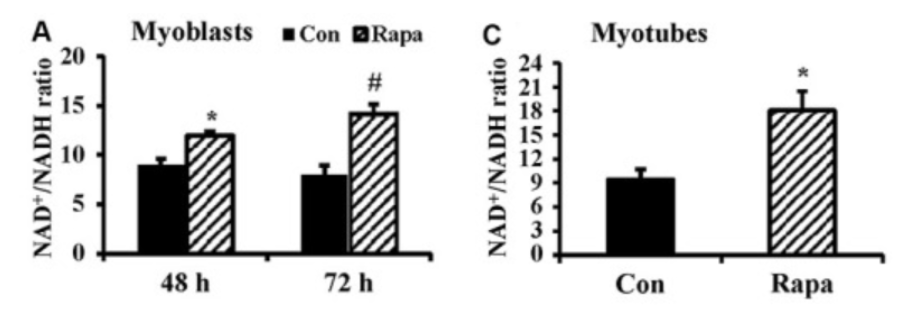
Rapamycin Promotes Cellular Production of Energy
NADH is known to transfer electrons to an ATP-producing enzyme within the mitochondria, cellular structures that manufacture energy-carrying compounds called adenine triphosphate (ATP). ATP is a direct source of energy for the cell that powers a multitude of cellular functions such as cell growth, metabolism, and survival.
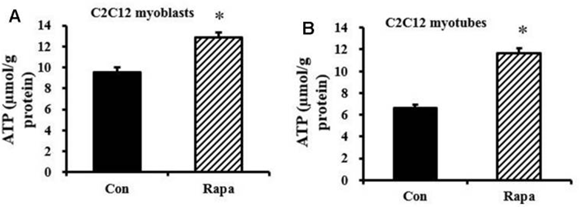
Subsequently, Baur and colleagues considered that the diminished NADH levels induced by rapamycin treatment could lower cellular ATP production, but their findings proved otherwise. Rather, ATP levels were elevated in myoblast and myotube cells after one day of rapamycin administration to cultured cells. Thus, the investigators hypothesized that the increases in ATP concentration could be explained by a rapamycin-induced cutback in demand for cellular energy.
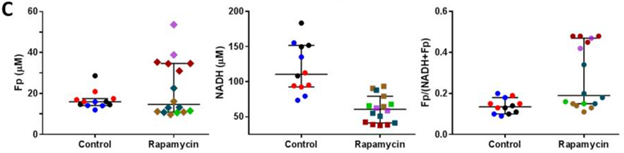
Rapamycin Improved Mouse Leg Muscle Metabolic Imbalance
The team of researchers examined aged mice leg muscles to determine if the results of mouse muscle cells could be applied to an entire organism. They administered 17-month-old aged mice with 2 mg/kg injections of rapamycin prior to utilizing an imaging approach to examine rapamycin’s effects on the NAD+/NADH ratio. Results showed no notable changes with treatment in a marker indicative for NAD+ concentrations (Fp) but found diminished NADH and elevated levels of a marker suggesting increased NAD+/NADH.
“Interestingly, in the presented study, we found significant increases in both NAD+/NADH ratio and ATP content in long-term cultured C2C12 myoblasts and myotubes after rapamycin treatment,” stated the researchers in their publication. “This suggests that energetic demand is decreased in the presence of rapamycin, rather than the capacity for ATP synthesis.” As a whole, the findings indicated that rapamycin does not inhibit mitochondrial ATP synthesis but rather that it decreases the demand for ATP that gets used in energy-consuming pathways.
Future Research to Uncover How Rapamycin Promotes Longevity
“This study provides new insight into the mechanisms by which rapamycin might influence the aging process to improve health and longevity among the aging population,” said the authors. Additional research is required to figure out how rapamycin leads to lower cellular energy consumption. Although the study demonstrates a limited understanding of how rapamycin may alter the aging process, it does indicate that rapamycin could be used to promote health and longevity in aging humans by targeting metabolic imbalances. Future studies are necessary to determine if rapamycin promotes comparable anti-aging effects in individuals.