Highlights
- A genome-wide CRISPR-Cas9-based screen was utilized by Wang et al. to locate genes that could affect cellular senescence.
- KAT7 was shown to be a promoter of senescence in stem cells of adult humans.
- Extension of mice life span and rejuvenation of premature aged progeroid humans cells resulted from inactivation of KAT7.
While we know that aging can be expedited through cellular senescence, a state of arrest in cell division, it is still unclear how this process functions. Our comprehension of the driving forces of cellular senescence is significant in developing therapeutics to stimulate anti-aging and promote longevity.
In a published article from the journal Science Translational Medicine, Wang and colleagues from the Chinese Academy of Sciences in Beijing sought out to locate genes that could affect cellular senescence. CRISPR, a common tool for gene-editing, was utilized to acquire DNA from human cells that modeled aging. This allowed for the location of genes that reduce cellular senescence upon deficiency. Screen results yielded KAT7, which upon inactivation, led to the restoration of human cells that exhibited premature aging. Furthermore, prolongation of life span was seen in both mice aging prematurely and normally when KAT7 was immobilized.
This study not only hones in on KAT7 as a potential target for the development of anti-aging therapeutics but also shows how increased longevity can be achieved through utilizing CRISPR-based gene editing. “Our study addy another example showing the possibility of using gene therapy for antagonizing aging and aging-related disorders,” said the authors in their article.
Slowing cellular senescence in humans
Almost all organisms decline in functionality upon aging, which is ultimately inevitable. Senescence in cells is not only a hallmark of aging but also a cardinal contributor to the aging processes. As tissues acquire more and more senescent cells upon aging, features associated with aged organisms can arise along with the development of arthritis and Alzheimer’s, which are common age-related diseases.
Cellular senescence is not just an indication of aging; this state of arrest in cell division has a causative role in aging organisms. For example, cellular senescence gradually increases upon liver degeneration, whereas reducing the level of cellular senescence in the liver weakens the manifestation of hepatic steatosis (or fatty liver disease). Therefore, the development of novel targeted therapeutics for treating aging-related disorders may be possible through halting or reversing cellular senescence.
While we’re aware of the changes in the human body that stem from aging, information on genes that are associated with aging have been derived from experiments that utilized model organisms with short life spans, such as fish, yeast, flies, and worms. Therefore, illuminating information on the genes that control aging in humans remains a crucial goal in the scientific community.
Screen identifies new human senescence-promoting genes
Through the utilization of CRISPR, Wang and colleagues were able to acquire two human cell types that displayed accelerated accumulation of senescent cells and delete small pieces of DNA, which allowed for the identification of genes that affect the aging process. The mutations found in these cells are linked to the following premature aging diseases: Hutchinson-Gilford progeria syndrome (HGPS) and Werner syndrome (WS). Of the genes that were identified to diminish the concentration of senescent cells through deficiency of those genes, KAT7 ranked the highest in its ability to inhibit cellular senescence in the two human cell models (WS and HGPS) of premature aging.
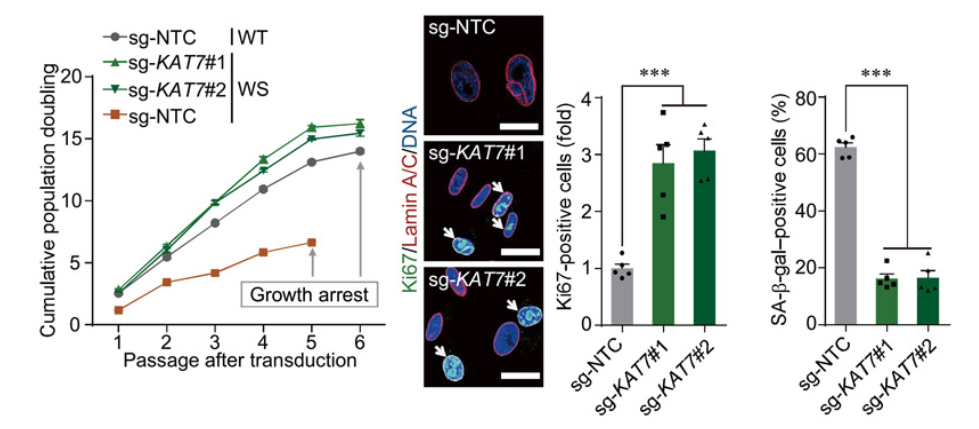
To determine whether KAT7 deficiency improved cellular senescence, the research team utilized CRISPR to silence the KAT7 gene in WS, HGPS, and replicative senescent adult stem cells. Wang and colleagues showed that ablation in each human cell model enhanced the potential of proliferation and improved features associated with aging. In addition, senescence models of oxidative stress, UV, and cancer were improved through ablation of KAT7. The presented results suggest the possibility of reducing cellular senescence in a variety of biological settings through depletion of KAT7.
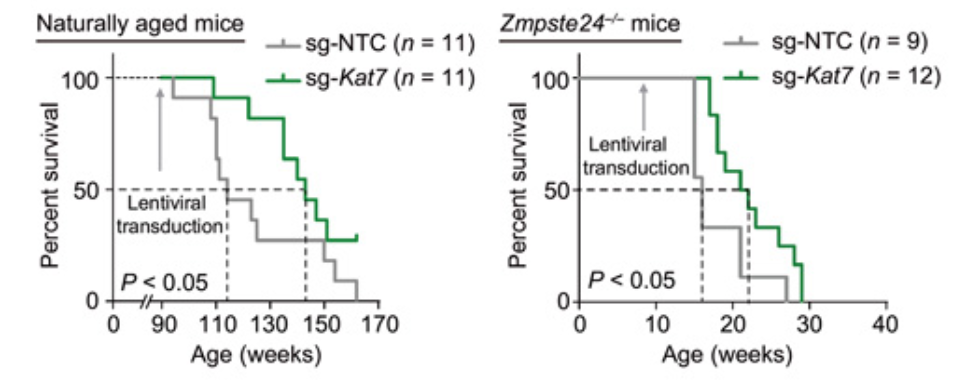
Wang and colleagues concluded their experiments by testing anti-aging CRISPR gene therapy on living organisms. Researchers attempted to elucidate the effects on naturally aged mice and prematurely aged mice that resulted from KAT7 inactivation through CRISPR. After CRISPR components were administered intravenously with the use of viruses, KAT7 was successfully inactivated and ultimately led to the improvement of cellular senescence in the liver, alleviation of liver aging, and extension of life span in both mice models. While the observed effects were promising, other organs failed to display these effects, and researchers concluded that this was likely due to the viral treatment method, which only occurred in the liver of mice. To further elucidate the function of KAT7 intervention and evaluate its safety, researchers need to further investigate the consequences in various cell types and specific organs that result from targeting KAT7.
Can CRISPR be used as an anti-aging treatment?
“Our study adds a layer of complexity to KAT7 function by revealing its important role in aging biology and pinpointing KAT7 as a new target for delaying aging and treating aging-associated disorders,” said the authors in the article. “In addition to unraveling the role of KAT7 in mediating aging, our screen identified additional senescence genes that might be targeted to ameliorate aging-related processes.”
Moreover, Wang and colleagues demonstrate how the inactivation of senescence genes through CRISPR-based gene editing can lead to human cell rejuvenation. Although this proposed therapeutic shows promise in combating aging, it’s far too early to formulate any conclusions on improving healthspan and longevity through the incorporation of CRISPR-based techniques.