Baur and colleagues from the University of Pennsylvania School of Medicine reported in a study published in Nature that a cellular transporter, SLC25A51, allows the entry of an essential cellular coenzyme into the cell’s power-generating structure, the mitochondria. The essential coenzyme, nicotinamide adenine dinucleotide (NAD+), has crucial roles in mitochondrial cellular metabolism, where it converts nutrients to cell energy. Low cellular NAD+ levels are a hallmark of aging, and scientists have linked lowered NAD+ levels with age-related diseases from Alzheimer’s disease to heart failure.
“We have long known that NAD+ plays a critical role in the mitochondria, but the question of how it gets there had been left unanswered,” stated Joseph A. Baur, Ph.D., an author of the study and an associate professor of Physiology at UPenn in a press release. “This discovery opens up a whole new area of research where we can actually manipulate – selectively deplete or add – NAD+ at a subcellular level, now that we know how it’s transported.”
The finding puts an end to the mystery of how NAD+ gets into mitochondria for the production of the energy molecule adenosine triphosphate (ATP) for cells. Scientists studying the matter had several ideas for the topic, including that mitochondria in mammals could not transport NAD+ but instead, NAD+ synthesis happens within mitochondria. Baur and colleagues considered SLC25A51 as a candidate transporter, though, since it’s classified as essential in screens of all genes and because it was a mitochondrial protein without a previously known function.
The evidence from the study showing that the transporter SLC25A51 dictates mitochondrial NAD+ levels came from tests done on human cells where the team reduced or eliminated protein levels of this transporter by genetic manipulations. They used NAD+ biosensors to measure NAD+ levels and confirmed a reduction in mitochondrial NAD+ levels in cells with a deficiency for the transporter. Moreover, increasing transporter protein levels increased mitochondrial NAD+ levels. Eliminating the transporter resulted in the loss of NAD+ from mitochondria, while NAD+ levels remained unchanged in the cell as a whole, indicating that the effects of this NAD+ transporter apply to mitochondria within cells specifically.
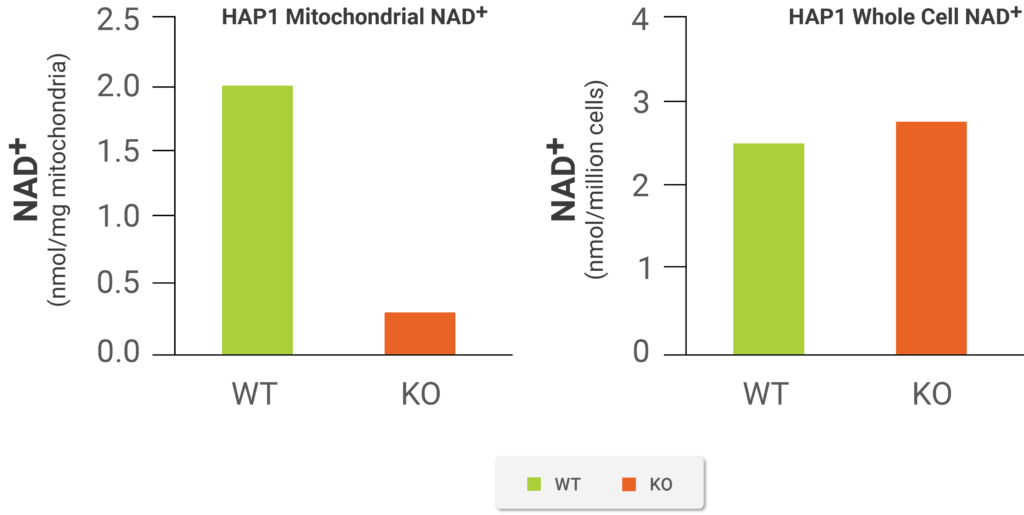
(Luongo et al., 2020 | Nature) Elimination of the SLC25A51 transporter reduced NAD+ levels in mitochondria of human cells but not in the cell as a whole. The scientists eliminated the protein levels of the SLC25A51 protein levels in the human cells with a genetic manipulation (KO). The graph on the left shows the data from the biosensor they used to measure NAD+ levels indicating that the genetic manipulation leading to SLC25A51 elimination (KO) reduced mitochondrial NAD+ content compared to the healthy (WT) cells. The graph on the right illustrates how NAD+ levels remained the same in the cell as a whole with elimination of the SLC25A51 transporter. Therefore, the transporter specifically affected entry of NAD+ into the mitochondria.
The study indicated that the presence of the SLC25A51 transporter impacts reactions in cells using NAD+ to convert nutrients into cell energy, a process called cellular respiration. Deficiency of or eliminating the transporter with genetic manipulation impaired the capacity for the occurrence of these energy-generating reactions. Restoring the transporter’s presence re-initiated cellular respiration for energy production.
In their study, the researchers went on to provide evidence suggesting the SLC25A51 transporter drives mitochondrial NAD+ uptake. To do so, they isolated mitochondria from cells with depleted or genetically-eliminated SLC25A51 that they incubated with supplemented NAD+. In mitochondria that lacked SLC25A51, incubation with a solution of supplemented NAD+ did not increase NAD+ content in mitochondria. When they restored the SLC25A51 presence, it led to the uptake of administered NAD+, thus indicating the critical role this transporter has in regulating mitochondrial NAD+ levels.
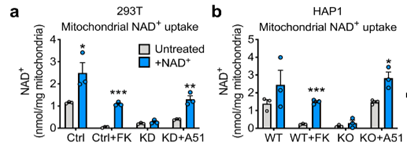
(Luongo et al., 2020 | Nature) The SLC25A51 transporter is required for NAD+ uptake in mitochondria. Figures (A) and (B) show results from two different human cell types, 293T and HAP1 cells. The grey bars on each graph represent mitochondrial NAD+ levels before treatment with NAD+, and the blue bars represent NAD+ levels in mitochondria after a 40 minute incubation with 1 mM NAD+. In both cell lines, deficiency or elimination of SLC25A51 results in significant inhibition of NAD+ uptake into the mitochondria. Restoration of the transporter with A51 adenovirus results in the restoration of mitochondrial NAD+ uptake. (A) In the 293 T, Ctrl represents standard cells with no alterations, Ctrl + FK represents treatment with an NAD+ inhibitor, KD represents SLC25A51 transporter deficiency, and KD + A51 represents SLC25A51 level restoration with a technique called adenovirus insertion. (B) In the HAP1 cells, WT represents standard cells with no alterations, WT + FK represents cells treated with an NAD+ synthesis inhibitor, KO represents cells without the SLC25A51 transporter, and KO + A51 represents restored SLC25A51 transporter expression levels.
“An approach to specifically alter the mitochondrial NAD+ pool is something many researchers have been looking for, so I would expect that we will see this gene targeted in a multitude of systems,” said Baur in the press release. “I think this is going to be a really valuable tool to help us better understand the function of mitochondrial NAD+ and its therapeutic potential.”
The finding that SLC25A51 is a transporter for mitochondrial NAD+ entry is important, because it could lead to the manipulation of mitochondrial NAD+ levels for disease treatment such as types of cancer. For instance, altering mitochondrial NAD+ levels could target cancers with heavy reliance on cellular respiration, the cellular reactions using NAD+ to convert nutrients to cellular energy. At the same time, increasing cells’ cellular respiration utilization could target other forms of cancer that rely on a different cellular energy-generating pathway called glycolysis. Manipulating NAD+ levels in mitochondria could therefore lead to significant clinical breakthroughs.