The roles of the vital molecule nicotinamide adenine dinucleotide (NAD+) in cells are organized and coordinated based on how it’s subdivided into different structures within cells. The molecule’s distribution inside the cells is critical to understanding NAD+’s impact on biological processes, diseases, and aging.
Investigators from the University of Texas at Austin and the University of Texas Southwestern Medical Center published a review in Trends in Biochemical Sciences where they broke down the evidence supporting how differing NAD+ levels are separated into distinct cell structures and how NAD+ synthesis modulation dynamically regulates signaling by determining subcellular NAD+ levels. They also discuss potential benefits to the cell of NAD+ compartmentalization and ways to measure NAD+ levels within different parts of the cell.
Every cell is made of a smorgasbord of molecules made by a complex series of reactions that convert sugars into metabolic precursors, molecules that can be used to generate energy or synthesize cellular building blocks, and NAD+ plays an essential role in this process. When cells lack NAD+, they can’t produce energy or cellular components, and even moderate reductions in NAD+ levels can limit cell functions that depend on NAD+.
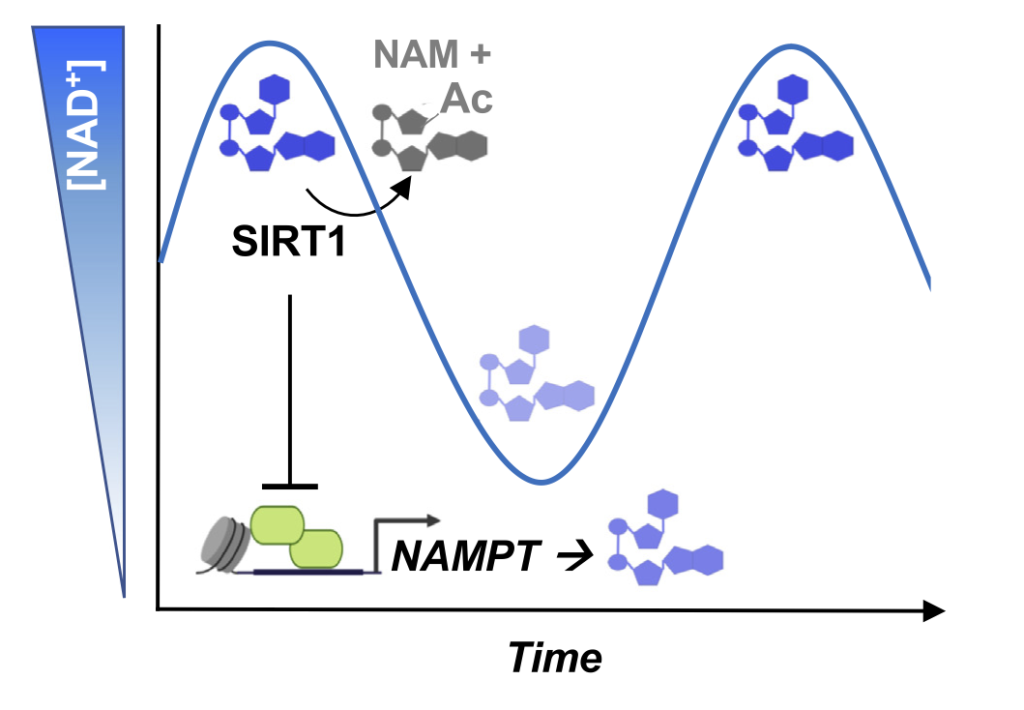
The varying NAD+ levels floating around freely in cells are controlled to precisely modulate signaling within cells. Dividing NAD+ into different cell components helps the cells with response timing, cell status communication, and the protection of crucial NAD+ pools.
Along these lines, regulating NAD+ levels in cells plays a crucial role in regulating biological outcomes. For example, NAD+ level fluctuations over time regulate rhythms of the body’s clock. Also, for fat cell development initiation, hormones or elevated sugar levels promote increased amounts of an enzyme called nicotinamide nucleotide adenylyltransferase 2 (NMNAT-2), resulting in diminished NAD+ levels in the nucleus, which then activates a gene program to turn precursor cells into fat cells. Finally, variable distributions of NAD+ within cells may protect and prioritize support for particularly critical cellular processes, like those in the mitochondria, in response to stress when other subcellular stores become depleted.
Given the importance of how NAD+ is subdivided in cells for key biological systems, changes to this process are drivers of disease. Even though direct findings of a role for NAD+ distribution’s disruption in certain diseases are limited, recent studies have begun to hint at its importance. For example, the same process with which NMNAT-2 regulates NAD+ partitioning also controls a genetic program for normal fat cell development, which is exploited by some brain cancer cells to control a genetic program for their growth. Many questions need to be addressed to get a complete picture of how NAD+’s distribution in cells is involved in cancer and aging in humans.
Determining the extent of NAD+ partitioning and NAD+’s dynamic modulation by its synthesis within cells as well as their impact on signaling pathways will be an area for rich future research. “We are only beginning to elucidate how the temporal and spatial compartmentalization of NAD+ contributes to its numerous biological roles,” said the researchers.
For these reasons, biological sensors — molecules that are capable of detecting chemicals that can be made by genetically modified cells — for NAD+ represent a promising approach for additions to the toolbox for measuring and studying the fluctuations in NAD+ levels within cells. For example, different sensors with sensitivity to NAD+ at varying ranges may reflect differences in the expected concentrations in various cellular milieus, cell types, and species. Also, the precise measurement of intracellular NAD+ in particular cells structures are currently lacking, and the possibility of focal NAD+ regulation at specific sites of DNA damage hasn’t been addressed.
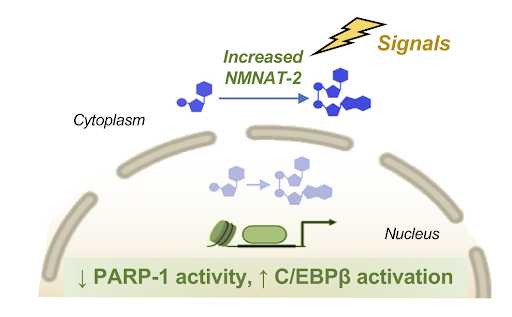
Spatial Regulation of NAD+ by Dynamic Compartmentalization. Hormones that induce the development of fat cells or elevated sugar levels promote an increase in levels of an enzyme called nicotinamide nucleotide adenylyltransferase 2 (NMNAT-2), resulting in increased cytoplasmic and decreased NAD+ levels in the nucleus. During the early stages of fat cell development, a decrease in free NAD+ levels in the nucleus limits the activity of enzymes called poly(ADP-ribose) polymerases (PARPs). For example, when the activity of a PARP-1 goes down, a program to turn precursor cells into fat cells that is dependent on a protein called C/EBPβ gets activated (Cambronne and Kraus Trends Biochem Sci. | 2020).
Since NAD+ is crucial for organisms in all domains of life for energy production and the generation of building blocks of cells, NAD+ regulation is integral to every discipline and field of study in biology and medicine. “Not only are its chemistry and mechanisms part of the history of biochemistry, but its broad and biological impact will undoubtedly also continue to bring together expertise across multiple disciplines in the future,” said the investigators. “The next wave of discoveries in NAD+ biology will be driven by collaborations among scientists with distinct skill sets.”